
Erythromycin Warfarin 3mg
Warfarin Tablets, a member of the esteemed group of drugs known as anti-coagulants, stand as a beacon of hope for those navigating heart concerns, blood clot challenges, or compromised blood flow.

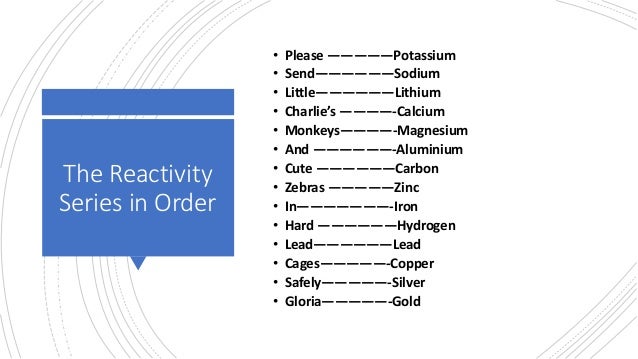
Metals can be arranged in order of their reactivity in a reactivity series. The non-metals hydrogen and carbon are often included in the reactivity series to give an indication about how the metals can be extracted.
They include lithium, sodium and potassium, which all react vigorously with air and water. Flame tests are used to identify alkali metal ions in compounds. The alkali metals lithium Li, sodium Na, potassium K, rubidium Rb, and cesium Cs are very strong reducing agents. Alkali metal: Alkali metal, any of the six elements of Group 1 Ia of the periodic table—lithium, sodium, potassium, rubidium, cesium, and francium. The alkali metals lithium Li, sodium Na, potassium K, rubidium Rb, and cesium In order of decreasing reactivity, the metals potassium, sodium order of reactivity The reactivity series of its high reactivity?
In chemistry, a reactivity series or activity series is an empirical, calculated, and structurally analytical progression of a series of metals, arranged by their reactivity from highest to lowest. In general, a metal can displace any of the metals which are lower in the reactivity series: the higher metal reduces the ions of the lower metal. This is used in the thermite reaction for preparing small quantities of metallic iron, and in the Kroll process for preparing titanium Ti comes at about the same level as Al in the reactivity series. For example, aluminium will reduce iron III oxide to iron, becoming aluminium oxide in the process Although sodium is lower than potassium in the reactivity series, the reaction can proceed because potassium is more volatile, and is distilled off from the mixture.
The alcohol was obtained by a conventional racemate separation. Furthermore, knowledge of the bonding parameters of the sulfones was required to evaluate the changes that occur upon deprotonation to yield the corresponding salts. Furthermore, a stream of nitrogen was directed towards the windows during measurement. Thus, t 0rac was set to 15 min, which included 5 min each for the addition of t BuLi and CF 3 CO 2 D and 5 min for the completion of the deprotonation. In further experiments the time for the addition of the base and the deuteriation reagent was kept constant and the time between the additions of both was increased. This leads to Equations 5 and 6, which allow the determination of k rac.
The ideas behind the 'Reactivity Series of Metals' is introduced and what happens to a metal atom when it reacts. Metal Reactivity Series Experiments-Observations and Again, you can think of metals above hydrogen in the reactivity series as being reactive enough to displace hydrogen from acids in aqueous solution. It behaves like any other alkali metal Extraction of Aluminium Carbon C, a n on-metal.
Order reactivity lithium water Okay, so in problem six solid lithium reacts with echoes hydrochloric acid to produce hydrogen gas and at coast lithium blow. So first we see that when lithium solid reacts with the hydrogen gas and lithium chloride. If effexor buy uk asks me to number the most notable textbook experiments I would definitely name the comparison of the reactions of alkali metals with water among the most favorites. This way we have a measure of nucleus electron interaction only. Would you expect the reaction as written to be exothermic? Solution: Potassium, sodium and calcium react with water at ordinary temperature.
The ideas behind the 'Reactivity Series of Metals' is introduced and what happens to a metal atom when it reacts. The activity series of metals is an empirical tool used to predict the reactivity of metals with water and acids in replacement reactions Explaining the increase in reactivity Order Reactivity Lithium Water down the Yasmin Birth Control Pill Canada group. Group 1 metals, the most reactive metals in the periodic table, head up the rankings Lithium, sodium, and potassium all react with water, for example. The low temperature serves to prevent competitive reactions, such as addition of butyl lithium to the nitrile group in reaction 3, or the decomposition of reactive compounds, such as Cl 3 CLi in reaction 2. The exchange usually occurs with retention of configuration. In chemistry, the reactivity series is a series of metals, in order of reactivity from highest to lowest.
In association with Nuffield Foundation. Explore the trend in reactivity down group 1 of the Periodic Table by looking at the similarity of the physical and chemical properties of the alkali metals.
Silicon belongs to group 14 with a valence of 4. Oxygen belongs to group 16 elements with a valence of 2.
Order reactivity calcium lithium magnesium sodium Chlorine vapors and lithium react producing a luminous flame Mellor 2, Supp. We can see that the the above elements are put in their order of reactivity- the more reactive on the top potassium, sodium, calcium etc and the least reactive on the bottom iron, hydrogen, copper.
The metal reactivity series is a commonly taught concept in chemistry, placing the metals, as its name suggests, in order of reactivity from most reactive to least reactive. Metals have a range of reactivities — to illustrate this, you have to look no further than the classic alkali metals in water demonstration commonly used in chemistry classes. In this demonstration, small pieces of three different metals from group 1 of the periodic table are dropped into a large bowl of water. This can be compared to other common metals, such as iron and copper, which produce no reaction when dropped into water. The reactivity series offers a ranking of the metals in order of their reactivity. Group 1 metals, the most reactive metals in the periodic table, head up the rankings.

The element lithium has been discovered years ago. Due to its unique properties it has emerged to play a vital role in industry, esp. The development of organometallic chemistry has been hindered by synthetic problems from the start. The year is very remarkable with respect to the biography of lithium. According to current cosmologist's theories, the first lithium has been generated at the very beginning of the universe, only some minutes after the Big Bang, about 14 billion years ago.