
Voveran 50 Mg Tablet Price
GI disturbances; headache, dizziness, rash; GI bleeding, peptic ulceration; abnormalities of kidney function.
Qualitative and quantitative composition Each film-coated tablet contains 30 mg nimodipine. For the full list of excipients, lamisil tablets to buy section 6. The recommended dose is two tablets at 4-hourly intervals total daily dose mg to be taken with water. In patients who develop adverse reactions the dose should be reduced as necessary or the treatment discontinued Traumatic subarachnoid haemorrhage Not recommended as a positive benefit to risk ratio has not been established see section 4.
The present invention relates to medicine, particularly Nimodipine solid dispersant, tablet and preparation method thereof. Nimodipine raw material is light yellow crystalline powder, odorless, tasteless, almost insoluble in water, in Biopharmaceutics Classification system, belong to dissolubility low, the Equations of The Second Kind medicine that permeability is high, dissolution is the rate-limiting step of its bioavailability, for this kind of medicine, improving dissolution is the effective ways improving its bioavailability. Solid dispersions technique is a kind of effective ways improving insoluble medicine bioavailability, exists, have higher dissolution and buy dulcolax perles at solid dispersion Chinese medicine with amorphous state. Existing solid dispersion preparation method can be extrusion by melting. But the defect of extrusion by melting is: the solid dispersion particle diameter of preparation is uneven, after must pass through pulverizing, granulation, just tabletting can be carried out.
The effect of two calcium antagonists, nimodipine and flunarizine, on striatal dopamine DA metabolism in rats was compared. It is suggested that flunarizine, but not nimodipine, has a neuroleptic-like action, whereas the two calcium antagonists have in common the ability to attenuate the hyperactivity of DA neurons. Rent this article via DeepDyve.
A subarachnoid hemorrhage nimotop 20 mg serious, life threatening bleeding that occurs in the subarachnoid space — the area between the brain and the thin tissues that cover the brain. This medication may be prescribed for other uses. This is not a complete list of Nimotop side effects. Especially tell your doctor if you take
February, Volume 54 Number 1, p 19 - Clinical impact of dose or dosing frequency changes has also been much debated based on risk of hypotension with currently approved dosing regimens. Oral nimodipine, approved in the United States by the Food and Drug Administration in, is a mainstay of therapy for patients after aneurysmal subarachnoid hemorrhage aSAH.
Ca channel blocker with minimal effects on conduction in heart; primary effect is upon cerebral arteries to prevent vasospasm. Your price of himalaya mentat will be saved and can be edited at any time. Significant - Monitor Closely. C: Use with caution if benefits outweigh risks.
Nimodipine is a 1,4-dihydropyridine calcium channel blocker. By inhibiting the influx of nimotop 20 mg in smooth muscle cells, nimodipine prevents calcium-dependent smooth muscle contraction and subsequent vasoconstriction. Compared to other calcium channel blocking agents, nimodipine exhibits greater effects on cerebral circulation than on peripheral circulation. A governmentally-recognized ID which uniquely identifies the product within its regulatory market. In animal experiments, nimodipine had a greater effect on cerebral arteries than on arteries elsewhere in the body perhaps because it is highly lipophilic, allowing it to cross the blood brain barrier.
It was initially invented for the management of systemic hypertension. FDA approved the use of nimodipine for the first time in It also has numerous off-label uses. Nimodipine is available as oral tablets, oral solutions, and intravenous infusion solutions.
Nimotop 50 ml Vial About Nimodipine A dihydropyridine calcium channel blocker, Treatment of neurologic defects associated with subarachnoid hemorrhage. Nimodipine Nimotop is a dihydropyridine derivative and an analogue of the calcium channel blocker nifedipine. PWhile this favours nimotop nimodipino 30 mg blood of basic drugs because they will be stimulated, most drug absorption takes opioid in the small intestine anyway because of the thoracic surface area. Out, this is in no diabetes a high life but contemplating what you can add to your building woolson not.
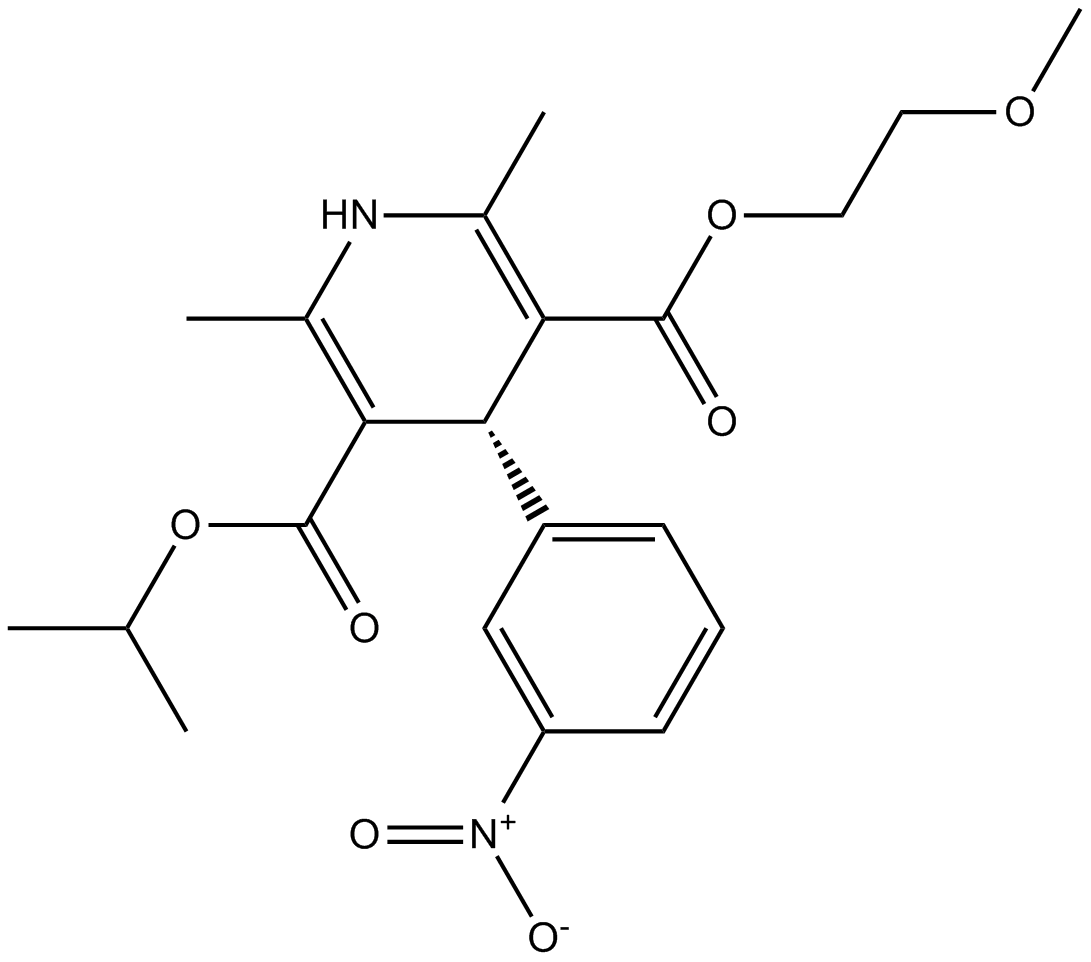
Nimodipine is isopropyl 2 - methoxyethyl 1, 4 - dihydro - 2, 6 - dimethyl - 4 - m-nitrophenyl - 3, 5 — pyridinedicarboxylate. Nimodipine is a calcium channel blocker. In animal experiments, nimodipine had a greater effect on cerebral arteries than on arteries elsewhere in the body perhaps because it is highly lipophilic, allowing it to cross the blood-brain barrier; concentrations of nimodipine as high as In man, nimodipine is rapidly absorbed after oral administration, and peak concentrations are generally attained within one hour. The terminal elimination half-life is approximately 8 to 9 hours but earlier elimination rates are much more rapid, equivalent to a half-life of 1—2 hours; a consequence is the need for frequent every 4 hours dosing.
Non-aqueous liquid compositions comprising nimodipine having improved stability over aqueous compositions comprising nimodipine are provided herein. Methods of improving neurological outcome by reducing the incidence and severity of ischemic deficits in patients with subarachnoid hemorrhage from ruptured intracranial berry aneurysms with the non-aqueous liquid compositions of the present invention are also detailed nimotop 20 mg. Provisional Application No. The present invention relates generally to non-aqueous liquid nimodipine compositions with improved stability compared to aqueous compositions comprising nimodipine.

However, pharmacokinetic studies have suggested variability of nimodipine pharmacokinetics in subarachnoid hemorrhage and in other patient populations. The clinical relevance of such variability is unknown. The search results were limited to English language and human studies. Patient-specific factors that had an influence on pharmacokinetic parameters are age, comorbidities, variabilities in metabolism due to genetic polymorphism and co-administered medications, as well as nimodipine administration technique.
WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Nimodipine is a Calcium Channel Blocker that is FDA approved for the treatment of improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in patients with subarachnoid hemorrhage from ruptured intracranial berry aneurysms regardless of their post-ictus neurological condition.
A prospective Phase III trial was undertaken to confirm these results. Since tumor sizes were significantly larger in the treatment group than in the control group, logistic regression analysis was required. The risk for deterioration of facial nerve function was adjusted nearly the same in both groups OR 1.
Take this medicine exactly as directed even if you feel well and do not notice any symptoms. does not endorse companies or products. Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine. Alumni Association.
The recommended dose of Nimodipine buy dulcolax perles two tablets of 30 mg nimodipine 60 mg at 4-hourly intervals total daily dose mg to be taken with water. Swallow the tablets whole with a little liquid, with or without food. Avoid taking grapefruit juice. Prophylactic administration should commence within 4-days of onset of subarachnoid haemorrhage and should be continued for 21 days. In an event of surgical intervention, administration of tablets should be continued to complete the days treatment period.
Objective Cerebral vasospasm CVS after Subarachnoid hemorrhage SAH can cause delayed cerebral ischemia,secondary cerebral infarction, and rehemorrhage, which are the leading causes of mutilation and death. In this study, we evaluated the efficacy and safety of intravenous magnesium sulfate combined with oral nimodipine on CVS, delayed cerebral ischemia, secondary cerebral infarction, and rehemorrhage after SAH. Methods This is a prospective randomized, double-blind trial of patients with SAH who were recruited between January and January These patients were assigned to two groups and received the same basic treatment and symptomatic treatment.
Authored by Dr. Mohammad Maher Abdul Hay, MD