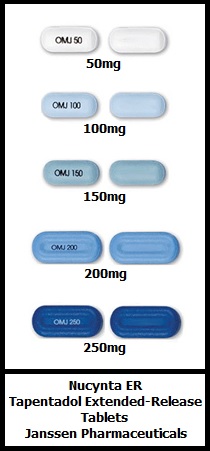
Nucynta Er 100mg
Diabetic peripheral neuropathy.
It is restricted for use in patients in whom morphine sulphate modified release has failed to provide adequate pain control or is not tolerated. In a meta-analysis, tapentadol prolonged-release demonstrated non-inferior efficacy, but superior gastro-intestinal GI tolerability compared to oxycodone modified-release MR tablets.
Sun Pharmaceutical Industries Ltd. Introduction Tydol 50 Tablet is a medicine used to treat moderate to severe acute pain in adults. It is used to treat many tapentadol 50mg such as headache, fever, period pain, toothache, and colds. It effectively alleviates pain when other treatments fail to relieve your pain. Tydol 50 Tablet may be prescribed alone or in combination with another medicine.
Now, I am a simple rural doctor. It gets metabolised in the liver by the cytochrome p enzymes into a few active chemicals. But here is the problem — the actual amounts of each of the active metabolites is a bit of a order tapentadol lottery.
Background Tapentadol is a newly available oral analgesic, approved by the FDA in for the management of moderate to severe acute pain in adults. Research Data The FDA approval was based on industry-coordinated, randomized controlled studies conducted in patients with osteoarthritis and after bunionectomy. In another single-dose study involving patients undergoing molar extraction, tapentadol mg demonstrated improved analgesia but higher sedation than 60 mg of oral morphine 4. Total daily doses greater than mg on the first day of therapy and mg on subsequent days have not been tested, nor has tapentadol been studied in children. It has not been comparatively studied against tramadol.
Acute and chronic pain is a major problem with repercussion in our society, causing impairment in the nucynta 50 mg street price of life of patients as well as socioeconomic losses, due to work absenteeism. Pain pharmacology: focus on opioids. Clin Cases Miner Bone Metab. Current considerations for the treatment of severe chronic pain: the potential for tapentadol.

Take tapentadol exactly as directed. Do not take more of it, take it more often, or take it in a different way than directed by your doctor. While taking tapentadol, discuss with your healthcare provider your pain treatment goals, length of treatment, and other ways to manage your pain. There is a greater risk tapentadol 50mg you will overuse tapentadol if you have or have ever had any of these conditions. Tapentadol may cause serious or life-threatening breathing problems, especially during the first 24 to 72 hours of your treatment and any time your dose is increased.
Fatal overdose may occur when opiates are combined with other depressants such as benzodiazepines, barbiturates, gabapentinoids, thienodiazepines, alcohol or other GABAergic substances. It is strongly discouraged to combine these substances, particularly in common to heavy doses. WARNING: Always start with lower doses due to tapentadol 50mg between individual body weight, tolerance, metabolism, and personal sensitivity. See responsible use section.
Tapentadol is a centrally-acting synthetic opioid which is structurally similar to tramadol. It is thought to bind to the mu opioid receptor and inhibit the reuptake of noradrenaline. In a trial of patients, tapentadol 50, 75 or mg every 4—6 hours was compared to immediate-release oxycodone 15 mg every 4—6 hours or placebo for acute pain after bunionectomy. Tapentadol and oxycodone were significantly better than placebo at relieving pain over the first 48 hours.
In, Janssen Pharmaceutical released a chemical entity known as tramadol Ultram. In, Janssen released a similar entity, tapentadol Nucynta, as a Schedule II analgesic that was the first new opioid entity with controlled substance status approved by the U. The drug was sold to DepoMed in April Although both compounds are mu opioid receptors agonists, they differ in their mu binding affinity: tramadol has a mu binding affinity 6, times less than that of morphine. This is presumably because of its dual mechanism—a mu receptor agonist and norepinephrine reuptake inhibitor.
If you forget to take a dose on time, take it as soon as you remember. If it is almost time for the next dose, do not take the missed dose. Return to your normal price of nucynta schedule. Do not take 2 doses of this medicine at one time.
Take this medicine only as directed by your doctor. Do not take more of it, do not take it more often, and do not take it for a longer time than your doctor ordered. This is especially important for elderly patients, who may be tapentadol 50mg sensitive to the effects of pain medicines.
Tapentadol SR is a centrally acting opioid analgesic that binds to the mu-opioid receptor. In addition it inhibits noradrenaline reuptake. Safety was not the primary endpoint of the trials, and the long-term safety profile tapentadol 50mg currently unknown.
Keep the medication in a place where others cannot get to it. Taking opioid tapentadol 50mg during pregnancy may cause life-threatening withdrawal symptoms in the newborn. Fatal side effects can occur if you use opioid medicine with alcohol, or with other drugs that cause drowsiness or slow your breathing. Limited visitation is permitted for hospitalized patients.
To assess the efficacy and safety of tapentadol IR for moderate to severe pain compared to oxycodone IR. Therefore, effective control of moderate to severe pain is a pressing unsolved issue in post-surgery patient care. Presently, opioids, such tapentadol 50mg oxycodone, morphine, and pethidine are considered to be the standard care to relieve moderate to severe pain 4. However, their utility is often limited by an array of gastrointestinal and central nervous system adverse effects including nausea, vomiting, constipation, dizziness, somnolence, and headache 5—8.
The MHRA reminds healthcare professionals that opioids co-prescribed with benzodiazepines and benzodiazepine-like drugs can produce additive CNS depressant effects, thereby increasing the risk of sedation, respiratory depression, coma, and death. Healthcare professionals are advised to only co-prescribe if there is no alternative and, if necessary, the lowest possible doses should be given for the shortest duration. Patients should be closely monitored for signs of respiratory depression at initiation of treatment and when there is any change in prescribing, such as dose adjustments or new interactions. If methadone is co-prescribed with a benzodiazepine or benzodiazepine-like drug, the respiratory depressant effect of methadone may be delayed; patients should be monitored for at least 2 weeks after initiation or changes in prescribing.
Authored by Dr. Linda Suriyakham, PHD