There is growing interest in the pharmacological treatment of obesity. Before, bupropion hcl generic were few weight loss medications approved by the FDA. The advent of Glucagon-like peptide-1 receptor agonists GLP-1 receptor agonists, with brand names like Wegovy and Ozempic, has attracted explosive media attention. It is not always easy to determine which medication is right for a particular individual. The market is also rapidly changing, so it is important to keep pace with the available options and their pros and cons.
If your care needs aren't being met, our care zyban generic cost is here and eager to help! Fully recyclable and made from galvanized steel. A generic may, however, differ in its inactive ingredients i.

Bupropion is a special antidepressant in the NDRI family. Norepinephrine and dopamine reuptake inhibitors NDRIs zero in on mood-affecting chemicals in the brain and help to regulate their levels to improve your symptoms. Serious adverse effects could include suicidal thoughts, anxiety, eye pain, instances of manic behavior, risk of seizures or seizure disorder in patients with eating disorders such as bulimia or anorexia, irregular heartbeat, and high blood pressure. Seek immediate medical advice from a healthcare professional if you experience these serious side effects. Learn about dealing with everything from relationship issues to burnout.
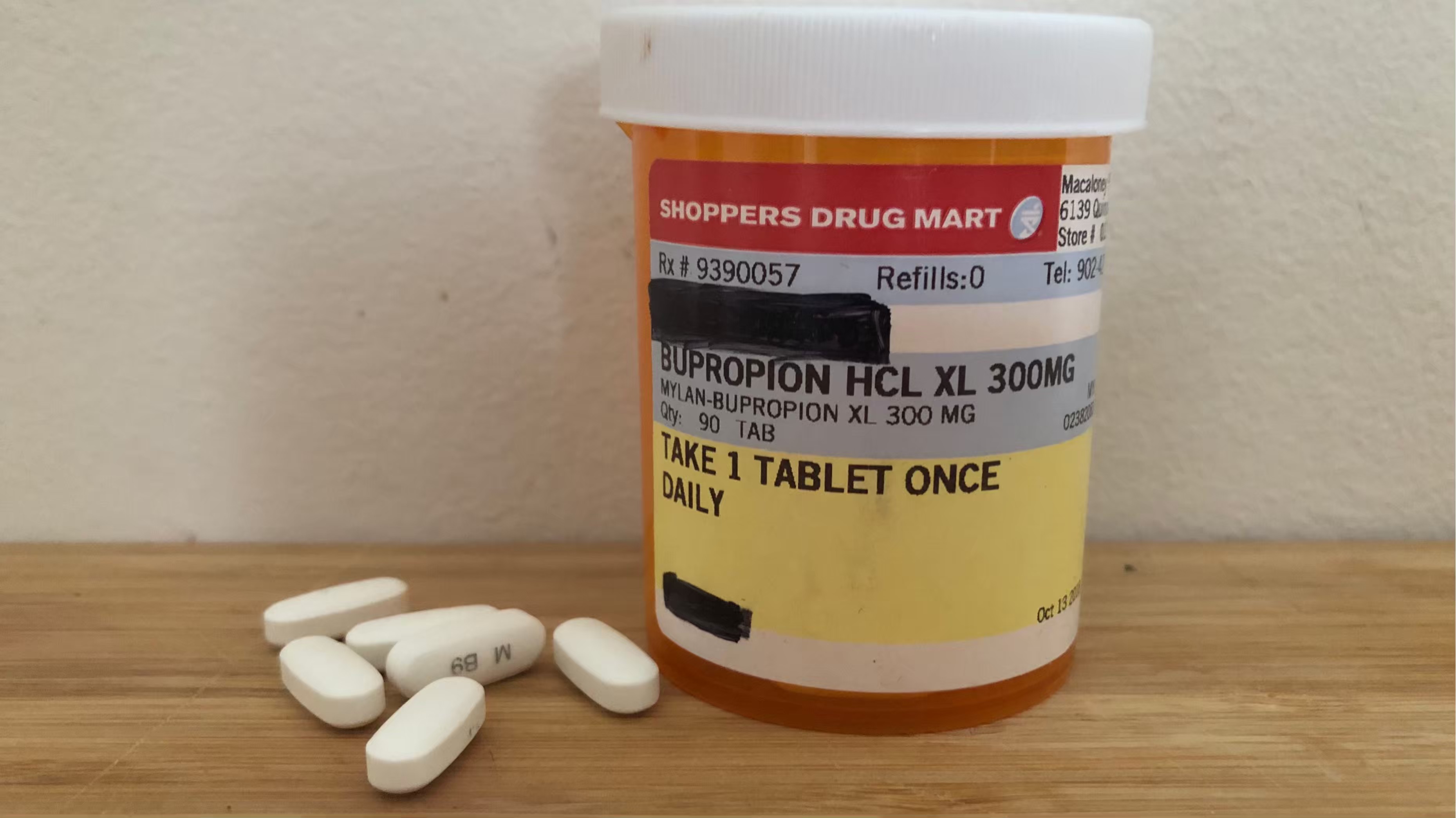
I have been taking the Generic kind. What do the Generic Bupropion look bupropion hcl generic I started taking the generic Wellbutrin 2xday, I had stomach aches, nausa, dizzy, mouth sores and funky taste in my mouth and profuse sweating. I had the non-generic before. This is the generic XL and I am doing fine!
Can u buy bupropion hcl generic best price online

All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases. Some patients who stopped smoking may have been experiencing symptoms of nicotine withdrawal, including depressed mood. However, some buy zyban online canada these adverse events occurred in patients taking bupropion who continued to smoke. Neuropsychiatric adverse events occurred in patients without and with pre-existing psychiatric disease; some patients experienced worsening of their psychiatric illnesses. Observe patients for the occurrence of neuropsychiatric adverse events.
Can i purchase bupropion hcl generic online safe
Each preparation was taken for 6 weeks. In, the results of the bioequivalence studies that the FDA had asked for came back Food and Drug Administration, There was definitely a problem with generic bupropion extended-release in the first few years after the medication went generic. The results of these studies came back in see below. Wellbutrin versus generic bupropion.
It is not intended or recommended for patients or other laypersons or as a substitute for medical advice, diagnosis, or treatment. These complaints about lower efficacy and more side effects with generic bupropion continued for several years. It asked other manufacturers of bupropion extended-release to conducted additional bioequivalence studies Woodcock et al. More data came from two sources: an FDA-funded clinical trial and FDA-mandated bioequivalence studies done by the generic manufacturers.
Patients must always consult a qualified health care professional regarding their diagnosis and treatment. Bioequivalence studies for the other bupropion extended-release generics In, the results of the bioequivalence studies that the FDA had asked for came bupropion hcl generic Food and Drug Administration, Bottom Line There was definitely a problem with generic bupropion extended-release in the first few years after the medication went generic.
Announcement of the availability of generic bupropion extended-release. The other manufacturers of bupropion extended-release mg tablets do not use the same technology as was used in Budeprion XL mg tablets Woodcock et al. Bioequivalence evaluation of innovator and generic bupropion XL products. Withdrawal of generic budeprion for nonbioequivalence.
Bioequivalence studies for the other bupropion extended-release generics. Medical Letter on Drugs and Therapeutics. I assume that they have learned their lesson.
As with all medications used in treatment, buprenorphine should be prescribed as part of a comprehensive treatment plan that includes counseling and other services to provide patients with a whole-person approach. Buprenorphine offers several benefits to those with OUD and to others for whom treatment in an Opioid Treatment Clinic is not appropriate or is less convenient. Buprenorphine is an opioid partial agonist. It produces effects such as euphoria or respiratory depression at low to moderate doses.
Where can i order bupropion hcl generic dose pack price
For 60 years, oral antidepressants have all been thought to work in bupropion hcl generic the same way. Until Auvelity — an oral treatment for adults with major depressive disorder MDD. While the exact way Auvelity works to treat depression is unclear, it affects certain brain chemicals thought to be involved in depression. Auvelity is a rapid-acting oral antidepressant.
This drug has boxed warnings. Boxed warnings alert doctors and patients about drug effects that may be dangerous. An extended-release drug is released into your system slowly over time.
The items in your order maybe shipped from any of the above jurisdictions. The products are sourced from bupropion hcl generic countries as well as those listed above. Rest assured, we only affiliate with our authorized dispensaries that procure product through reliable sources. Bupropion HCL affects the balance of brain chemicals thus treating symptoms. The typical dosage prescribed to patients is a daily dose of mg and do not exceed mg daily.
Where can u buy bupropion hcl generic shipping cost
All FDA black box warnings are at the end of this fact sheet. Bupropion is an antidepressant medication that works in the brain. It is approved for the treatment of major depressive disorder MDD, seasonal affective disorder SAD, and to help people quit smoking smoking cessation. SAD is a type of depression that occurs mainly during the autumn-winter season. They should be clear about the limits of the research around that medication and if there are any other options.
Actavis Group, the international generic pharmaceuticals company, recently announced that it has received approval from the U. Distribution of the product will commence immediately. Bupropion Hydrochloride extended-release tablets XL, available in mg strength, are the generic equivalent of Wellbutrin XL R for the treatment of major depressive disorder. This approval also highlights Actavis Group's focus and expertise in bringing complex controlled-release technologies to the marketplace.
Learn about the medical, dental, pharmacy, bupropion hcl generic, and voluntary benefits your employer may offer. It is important that you keep taking each dose of this medicine on time even if you are feeling well. If you forget to take a dose on time, take it as soon as you remember.
Bupropion is an antidepressant used to treat major depressive disorder and seasonal affective disorder. You should not take bupropion if you are allergic to it, or if you have Do not use an MAO inhibitor within 14 days before or 14 days after you take bupropion. A dangerous drug interaction could occur. MAO inhibitors include isocarboxazid, linezolid, phenelzine, rasagiline, selegiline, and tranylcypromine.

Impax requested that the Agency withdraw approval of Budeprion XL mg extended-release tablets. FDA does not anticipate a drug shortage. FDA also announced last week their analysis of studies to assess the bioequivalence of marketed bupropion HCl ER mg tablets provided by the three other manufacturers currently on the market: Actavis, Mylan Inc. The bioequivalence study results from the mg strength were extrapolated to establish bioequivalence of the mg product. This methodology was based on FDA's guidance at the time the products were approved.